electron affinity of nitrogen|electron affinity of nitrogen family : Tagatay August 11, 2023. Periodic table with electron affinity values is shown above. The values of electron affinity are given in kJ/mol. Values in parentheses ( ) are predicted values. Electron affinity is the amount of . Given a huge integer value n, find the largest integer value x such that x <= n and all the digits of x are prime. Examples: Input : n = 45 Output : 37 37 is the largest number smaller than or equal to with all prime digits.Input : n = 1000 Output : 777 Input : n = 7721 Output : 7577 Input : n = 7221 Output : 5777 We know that the prime digits are .One warranty for eligible devices you own now, and the ones you buy in the future. Covers repair, replacement, or reimbursement for eligible devices. . uBreakiFix Cobb County: 3605 Sandy Plains Rd Suite 125, .
PH0 · order of electron affinity
PH1 · least electron affinity
PH2 · electron affinity vs electronegativity
PH3 · electron affinity values
PH4 · electron affinity symbol
PH5 · electron affinity of nitrogen family
PH6 · electron affinity in periodic table
PH7 · electron affinity chart
PH8 · Iba pa
Ang Kalikasan at ang Tao [Walang Pinagkakaiba] Talumpati ni Miho “Ang Diyos ay nilikha tayong lahat ng pantay-pantay” Sa aking mga kaklase, aking mga minamahal na guro at sa ginagalang nating lahat na mga HURADO, Magandang Hapon po sa inyong lahat.
electron affinity of nitrogen*******August 11, 2023. Periodic table with electron affinity values is shown above. The values of electron affinity are given in kJ/mol. Values in parentheses ( ) are predicted values. Electron affinity is the amount of .
• Janousek, Bruce K.; Brauman, John I. (1979), "Electron affinities", in Bowers, M. T. (ed.), Gas Phase Ion Chemistry, vol. 2, New York: Academic Press, p. 53.• Rienstra-Kiracofe, J.C.; Tschumper, G.S.; Schaefer, H.F.; Nandi, S.; Ellison, G.B. (2002), "Atomic and molecular electron affinities: Photoelectron experiments and theoretical computations", Chem. Rev., vol. 102, no. 1, pp. 231–282, doi:10.1021/cr990044u, PMID 11782134.
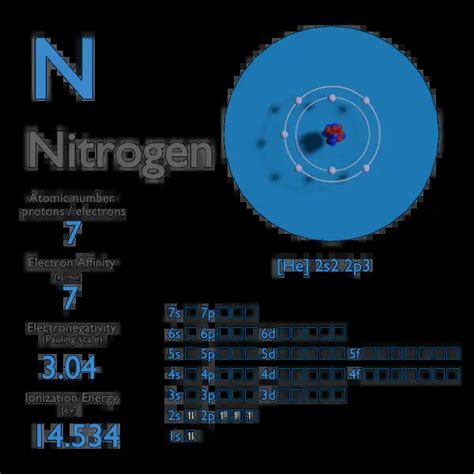
Nitrogen is unique in that it has an electron affinity of approximately zero. Adding an electron neither releases nor requires a significant amount of energy: N(g) +e− → N−(g) . Electron affinity of Nitrogen is 7 kJ/mol. In chemistry and atomic physics, the electron affinity of an atom or molecule is defined .
Electron affinity is defined as the change in energy (in kJ/mole) of a neutral atom (in the gaseous phase) when an electron is added to the atom to form a negative . Electron Affinity of Nitrogen is 7 kJ/mol. Electronegativity of Nitrogen is 3.04. First Ionization Energy of Nitrogen is 14.5341 eV. Electron Affinity. In chemistry and .Electron configurationThe arrangements of electrons above the last (closed shell) noble gas. Melting pointThe temperature at which the solid–liquid phase change occurs. Boiling .
When studying the effects of electron filling in chemistry, I was studying why energy is needed to add an electron to a nitrogen atom i.e. electron affinity. Nitrogen already has .The electron affinity ( Eea) of an atom or molecule is defined as the amount of energy released when an electron attaches to a neutral atom or molecule in the gaseous state .Electron Affinity (kJ/mol) Electron Configuration: H: 72.8 : 1s 1: He <0 : 1s 2: Li: 59.8 [He] 2s 1: Be <0 [He] 2s 2: B: 27 [He] 2s 2 2p 1: C: 122.3 [He] 2s 2 2p 2: N <0 [He] 2s 2 2p 3: O : 141.1 [He] 2s 2 2p 4: F: 328.0 [He] 2s 2 .Low electron affinity. iii. Low ionisation energy. iv. High non-metallic character. Assertion :Fluorine has a less negative electron affinity than chlorine. Reason: There is the relatively greater effectiveness of 2p− electrons in the small F atom to repel the additional electron entering the atom than to 3p− electrons in the larger Cl atom.
Since we typically expect electron affinity to be negative except for certain exceptions like Nitrogen, this is one of those exceptions. Nitrogen's electron configuration is 1s^2 2s^2 2p^3, so all three p orbitals have one electron each. Since electron affinity is associated with the "love" for acquiring another electron, and the new electron would be .
In addition, the relatively large magnitude of the electron affinity of the lighter pnicogens enables them to form compounds in the −3 oxidation state (such as NH 3 and PH 3), in which three electrons are formally added to .The table shows the first and second electron affinities for atoms of nitrogen and phosphorus. Why is the change in energy for both electron affinities lower for atoms of phosphorus than for atoms of nitrogen? [A] An atom of phosphorus has fewer electrons in its outer valence shell than an atom of nitrogen, and so there is less repulsion from the .
The key to why the electron affinity of nitrogen is actually negative lies with two factors. effective nuclear charge; electron configuration; As you know, electron affinity tells you how much energy is relesed (hence the negative sign) when one mole of electrons is added to one mole of atoms in the gaseous state.electron affinity of nitrogen electron affinity of nitrogen family(ii) Similar electronic configuration is repeated after intervals of 2, 8, 8, 18 and 32. (iii) Al 2 O 3 is an amphoteric oxide. (iv) On moving horizontally across a period, number of valency electrons increases from one to eight. (v) All members of zero group are non-metals. (vi) The elements with higher electron affinity have higher ionization .The electron affinity is the potential energy change of the atom when an electron is added to a neutral gaseous atom to form a negative ion. So the more negative the electron affinity the more favourable the electron addition process is. Not all elements form stable negative ions in which case the electron affinity is zero or even positive.Nitrogen is unique in that it has an electron affinity of approximately zero. Adding an electron neither releases nor requires a significant amount of energy: . The electron affinity (EA) of an element is the energy change that occurs when an electron is added to a gaseous atom to give an anion. In general, elements with the most negative .
electron affinity of nitrogenElectron Affinity: Electron affinity is defined as the change in energy that only occurs when electrons are added to any gaseous atom. The electron affinity value always increases when we go from left to right in a period. Electron affinity can be positive as well as negative. If electron affinity is negative, it means it's giving off energy.The electron affinity ( EA E A) of an element E E is defined as the energy change that occurs when an electron is added to a gaseous atom: E(g) +e− → E−(g) energy change=EA (8.5.1) (8.5.1) E ( g) + e − → E ( g) − energy change= E A. Unlike ionization energies, which are always positive for a neutral atom because energy is required . Nitrogen has very low electron affinity because of its half filled orbitals. The nitrogen is fairly stable atom than adjacent atom carbon in the periodic table. Thus the electron affinity falls from carbon to nitrogen. C: 1s 2 2s 2 2p 2: EA 1 = -122 kJ mol-1: N: 1s 2 2s 2 2p x 1 2p y 1 2p z 1:

Nitrogen -. N: properties of free atoms. Nitrogen atoms have 7 electrons and the shell structure is 2.5. The ground state electron configuration of ground state gaseous neutral nitrogen is [ He ]. 2s2. 2p3 and the term symbol is 4S3/2. Schematic electronic configuration of nitrogen. The Kossel shell structure of nitrogen.
These electron affi nities are in units of kJ?mol21, and they have been sum-marized from J. E. Huuhey et al., Inorganic Chemistry, 4th ed. (New York: HarperCollins, 1993). The 1st electron affi nity represents the energy required for the process: X(g) 1 e2 S X2 (g) while that of the 2nd electron affi nity represents that for: X2(g) 1 e2 S X22 (g) Also, oxygen’s electron affinity is greater than nitrogen because oxygen is more electronegative than nitrogen and hence it has more tendency to take electrons. Additional information:When one electron is added to the atom (in gaseous state), the energy change is called first electron affinity and this gives a negative charge to the . Nitrogen is unique in that it has an electron affinity of approximately zero. Adding an electron neither releases nor requires a significant amount of energy: . The electron affinity (EA) of an element is the energy change that occurs when an electron is added to a gaseous atom to give an anion. In general, elements with the most negative .
Electron affinities are the negative ion equivalent, and their use is almost always confined to elements in groups 6 and 7 of the Periodic Table. The first electron affinity is the energy released when 1 mole of gaseous atoms each acquire an electron to form 1 mole of gaseous 1- ions. This is more easily seen in symbol terms.
A negative electron affinity value denotes that upon the addition of an electron, the release of energy occurs. Less negative electron affinity means less energy is released and vice versa. For nitrogen, its electron affinity is less negative than that of carbon and nitrogen. This can be explained on the basis of its electronic configuration.electron affinity of nitrogen familyElectron Affinity. The electron affinity (EA) of an element E is defined as the energy change that occurs when an electron is added to a gaseous atom or ion: [latex]E_{(g)}+e^- \rightarrow E^-_{(g)} \;\;\; \text{energy change=}EA \label{7.5.1}[/latex] Unlike ionization energies, which are always positive for a neutral atom because energy is required to .
Asya Premier Suites is located at Boracay, Aklan, Philippines. It offers premier and executive suites, all of which have a panoramic vistas of the sea, and is equipped with Cable TV with DVD player, mini-bar, and NDD/IDD phone service. Some of its amenities include private shuttle speedboat and van, nanny & butler service, a fitness center, and .
electron affinity of nitrogen|electron affinity of nitrogen family